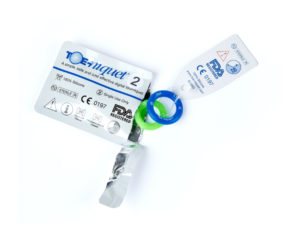
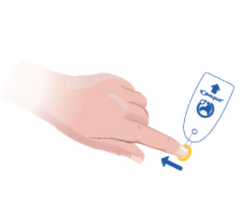
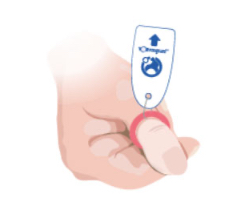
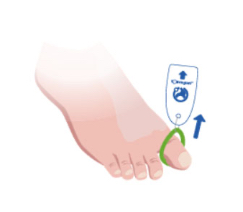
Toe-niquets™ are Single Use disposable ‘elastic-ring’ tourniquet devices designed to stop blood flow in digits of the hands and feet (fingers and toes) for use during surgery, trauma or first aid. Toe-niquets™ are easily applied by rolling over the tip of any digit.
Toe-niquets™ allow you to exanguinate (remove) blood from a digit and stop further blood flow at the point of application. Treatment may continue safely, quickly and easily without the risks of visual or physical obstructions associated with bleeding.
Toe-niquets™ are used during surgery, trauma or first aid by Medical Doctors, Surgeons, Surgical Theatres, Emergency Room Facilities and Podiatrists worldwide. They are supplied sterile ready for use in any clinical or surgical environment.

Easy Use – Sterile Packaging
Size 1
TOE-5G1
8mm and 11mm
Size 2
TOE-5G2
14mm and 17mm




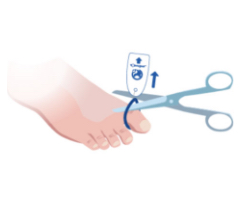
Conform to local, national and international clinical protocols and guidelines.

Pull Tag, Hypoallergenic
4 sizes (S, M, L, XL)
2 sets (S&M, L&XL)
Colour Coding & Strong
Optimal Elasticity Vs Compression Ratio
Latex free Medical Grade Silicone
Increased intra operative options
Less Stock for Distributors and Users
Improved Safety & Easier to Removal
Easy application, reduced tissue trauma